T-lineage acute lymphoblastic leukemia (ALL) is an aggressive cancer comprising of diverse subtypes that are challenging to stratify using conventional immunophenotyping and have historically exhibited poor treatment outcomes in response to cytotoxic chemotherapy ( Nguyen, K et al. Leukemia 2008). Early T-cell Precursor ALL (ETP-ALL) represent a distinct subtype that displays a developmental block at the earliest stages of T-cell commitment, accompanied by the aberrant expression of myeloid and stem cell markers ( Coustan-Smith, E. et al. Lancet Oncol. 2009). The classification can be further complicated by T/Myeloid Mixed phenotype acute leukemia (T/My-MPAL), a rare and aggressive malignancy characterized by blasts with both T-cell lymphoid and myeloid markers ( George, B. S. et al. Biomedicines 2022). Accurate clinical diagnosis of T-lineage ALL is hindered by phenotypic variations among its subsets. Nevertheless, the precise diagnosis holds critical importance as drug sensitivity in preclinical models of T-lineage ALL is closely linked to the differentiation state, such as the sensitivity of ETP-ALL to the BCL-2 inhibitor venetoclax ( Chonghaile, T. N. et al. Nat Cancer 2021). This highlights the critical need to identify consistent phenotypic traits associated with unique therapeutic vulnerabilities to effectively tailor therapy to individual patients.
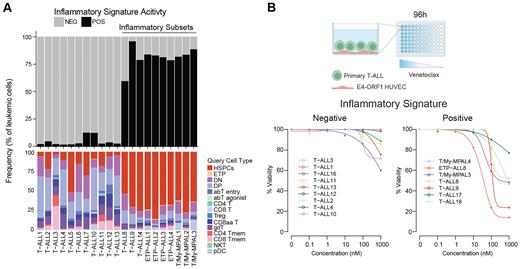
To gain insights into subset-specific therapeutic vulnerabilities, we performed an integrative multiomic analysis of bone marrow (BM) samples (n=21) from newly diagnosed T-lineage ALL patients, including T-cell ALL (T-ALL), ETP-ALL, and T/My-MPAL. Leveraging cellular indexing of transcriptomes and epitopes in conjunction with T-cell receptor sequencing, we identified a distinct subset of patients spanning all three subtypes characterized by the high frequency of leukemic cells that most closely resemble healthy hematopoietic stem and progenitor cells (HSPC) by Symphony ( Kang, J. B. et al. Nat Commun 2021) query mapping, indicative of an earlier stage of differentiation arrest ( Figure 1A bottom). Pathway analysis revealed an inflammatory signature in patients with high frequencies of stem-like cells suggestive of an inflammatory microenvironment akin to high-risk AML ( Lasry, A. et al. Nat Cancer 2023), which we refer to as inflammatory T-lineage ALL. Next, we mined our multiomic data to generate a comprehensive geneset for inflammatory T-lineage ALL scoring in single cells and bulk samples ( Figure 1A top). Interestingly, Olink cytokine array analysis uncovered upregulation of production of cytokines IL-1β, IL-18, and/or OSM in inflammatory T-lineage ALL samples. Thus, this comprehensive multiomic analysis identified a subset of T-lineage ALL patients characterized by early differentiation arrest and inflammatory signatures.
To explore the relationship between inflammatory T-lineage status and clinical response, we utilized the ssGSEA algorithm to score activity of our inflammatory signature in bulk RNA-sequencing data from the TARGET T-lineage ALL dataset (n=265, 74/265 inflammatory) ( Liu, Y. et al. Nat Genet 2017) and the Tran et al. study (n=27, 17/27 inflammatory) ( Tran, T. H. et al. Blood Adv 2022). We found a significant association between high inflammatory ssGSEA score and elevated MRD. To uncover the inflammatory T-lineage ALL mutational landscape, we performed whole exome sequencing on our study cohort, and compiled the reported mutations in the TARGET dataset. Interestingly, inflammatory T-lineage ALL patients, as predicted by inflammatory signature activity, had a significantly higher prevalence of mutations affecting cytokine signaling ( NRAS, JAK3), chromatin remodeling genes ( EZH2), and the WT1 oncogene. These analyses demonstrate a significant relationship between inflammatory status, genomic lesions, and MRD.
Finally, we demonstrate that inflammatory, but not non-inflammatory, T-lineage ALL cells are significantly more sensitive to BCL-2 inhibitor venetoclax ex vivo (Figure 1B).Overall, our study has identified a new subtype of T-lineage ALL defined by an early arrest in T-cell development, inflammatory signaling, a unique mutational landscape, sensitivity to venetoclax, and an association with adverse clinical parameters.
Disclosures
Tierens:BD Biosciences: Honoraria, Speakers Bureau. Loghavi:Caris Diagnostics: Consultancy; Blueprint Medicine: Consultancy; Abbvie: Consultancy; Gerson Lehrman Group: Consultancy; QualWorld: Consultancy; Guidepoint: Consultancy; Recordati/ EUSA Pharma: Consultancy; Daiichi Sankyo: Consultancy; Astellas: Research Funding; Amgen: Research Funding; Abbvie: Current equity holder in publicly-traded company. Dick:Celgene/BMS.: Research Funding; Graphite Bio: Consultancy, Membership on an entity's Board of Directors or advisory committees; Trillium Therapeutics Inc/Pfizer: Patents & Royalties: Trillium Therapeutics.